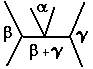
|
|
|
|
|
|
|
1. Get acquainted with Fe-Fe3C phase diagram.
2. Study the effect of the eutectoid invariant reaction on steel
microstructure.
3. Differentiate between eutectoid, hypo-eutectoid, and hyper-eutectoid
microstructures.
As discussed in the previous report, invariant reactions are those reactions accompanied by certain phase transformation and microstructural changes. These reactions happen at a given temperature and composition, giving them the name of a "zero degree of freedom'' reaction. Yet, with varying the content of the alloying element, it is possible to undergo invariant reactions, giving a certain amount of the "invariant" phase(s). In this report, the focus is upon the eutectoid reaction. In this reaction, a solid solution phase transforms into a 2-phase solid solution structure as indicated in the following equation and figure (1).
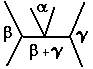

As shown in the Fe-Fe3C diagram, the iron iron carbide system
experiences the eutectoid reaction when the
gamma (austenite) phase transforms into alpha (ferrite) and Fe3C
(cementite) phases. In fact, such transformation happens
under certain conditions regarding the cooling rates, whether it is air
or furnace cooled. This affects the microstructure of the produced eutectoid
phases. In addition, increasing and decreasing the carbon content changes
the amount of eutectoid phase
Samples are made out of Fe-Fe3C. Four specimens with different
carbon content were investigated as follows:
-Specimen 1: 0.35% C
-Specimen 2,3 : 0.8% C with one furnace-cooled
and another air-cooled
-Specimen 4: 1.3% C
Etching:
-Specimen 1: etching for 15 seconds in
2% Nitric acid in alcohol (nital) solution.
-Specimen 2 &3: grinding and polishing
with diamond paste and finishing with a 1 micron grade, followed by etching
with the 2% nitric acid in alcohol solution.
-Specimen 4: Similar to 1
Optical Microscope
--The following sites and books contain useful information and images for several articles that we have encountered in this experiment. Make sure to check them.
Kalpakjian, Serope. Manufacturing Process for Engineering Materials. Addison Wesley, 3rd Ed., 1997.
Smith, William F. . Principles of Materials Science and Engineering. McGraw Hill, 3rd Ed., 1996. (p. 128-132)
Academic Page for a similar experiment¶¶¶ http://compfab.me.lsu.edu/me3701/experiments/metallography/expt6.htm
Academic Page for a similar experiment¶¶¶ http://compfab.me.lsu.edu/me3701/experiments/metallography/expt7.htm